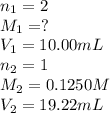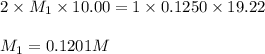Chemistry, 11.03.2020 18:35 genyjoannerubiera
You are using a standardized 0.1250 M solution of sodium hydroxide to determine the concentration of sulfuric acid. After pipetting 10.00 mL of the H2SO4 into your flask you find that you use 19.22 mL of your NaOH solution to reach the endpoint. No, wait... I mean What is the concentration of the sulfuric acid? H2SO4 + 2NaOH ---> Na2SO4 + 2HOH

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
You are using a standardized 0.1250 M solution of sodium hydroxide to determine the concentration of...
Questions


Mathematics, 22.10.2021 20:30


Physics, 22.10.2021 20:30


English, 22.10.2021 20:30







Chemistry, 22.10.2021 20:30


Mathematics, 22.10.2021 20:30

Mathematics, 22.10.2021 20:30


Mathematics, 22.10.2021 20:30

Chemistry, 22.10.2021 20:30

Chemistry, 22.10.2021 20:30

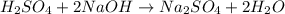
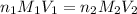
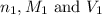 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 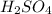
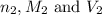 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.