
Chemistry, 11.03.2020 17:49 tylerkitchen44
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below?
2c2H6(g)+7O2(g)--->4CO2(g)+6H2O( g)
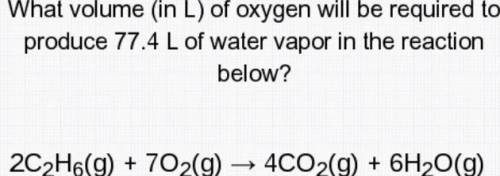

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below...
Questions

Mathematics, 04.11.2019 03:31

History, 04.11.2019 03:31


Biology, 04.11.2019 03:31


Business, 04.11.2019 03:31




Mathematics, 04.11.2019 03:31

Geography, 04.11.2019 03:31



History, 04.11.2019 03:31

History, 04.11.2019 03:31

Mathematics, 04.11.2019 03:31


Mathematics, 04.11.2019 03:31

English, 04.11.2019 03:31



