
Chemistry, 11.03.2020 13:33 lildanielmabien
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to the following equation.
Al2O3(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Group of answer choices
72.9 g
155 g
65.4 g
16.3 g
32.6 g

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Order the following from smallest to largest atom, electron, quark, proton, neutron, molecule, nucleus
Answers: 1

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
Find the mass of AlCl3 that is produced when 25.0 grams of Al2O3 react with excess HCl according to...
Questions


Mathematics, 17.10.2019 22:50





Computers and Technology, 17.10.2019 22:50



Mathematics, 17.10.2019 22:50





English, 17.10.2019 22:50

History, 17.10.2019 22:50




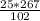 = 65.4 g of AlCl₃.
= 65.4 g of AlCl₃.

