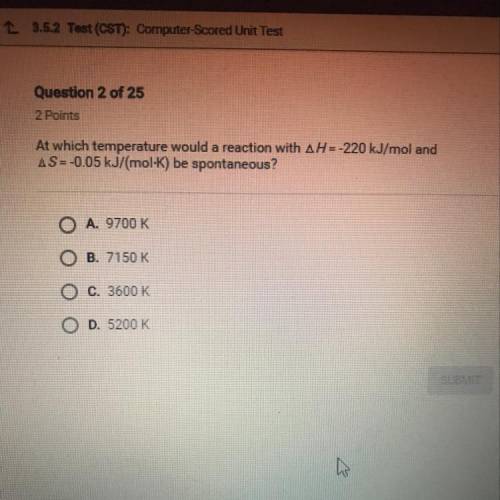
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontan...

Chemistry, 11.03.2020 09:51 michaelchavez6959127
At which temperature would a reaction with AH = -220 kJ/mol and
AS=-0.05 kJ/(mol-K) be spontaneous?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1
You know the right answer?
Questions



History, 28.08.2019 00:30



Biology, 28.08.2019 00:30

Mathematics, 28.08.2019 00:30

Biology, 28.08.2019 00:30

History, 28.08.2019 00:30

Social Studies, 28.08.2019 00:30

English, 28.08.2019 00:30


English, 28.08.2019 00:30

Biology, 28.08.2019 00:30




Mathematics, 28.08.2019 00:30


History, 28.08.2019 00:30



