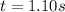Chemistry, 11.03.2020 06:13 dianacastro8298
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long, in seconds, would it take for the concentration of A A to decrease from 0.650 M 0.650 M to 0.380 M?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 30...
Questions




Law, 30.11.2020 21:30


Mathematics, 30.11.2020 21:30

Mathematics, 30.11.2020 21:30




Mathematics, 30.11.2020 21:30


History, 30.11.2020 21:30

Mathematics, 30.11.2020 21:30

Mathematics, 30.11.2020 21:30


Chemistry, 30.11.2020 21:30

Mathematics, 30.11.2020 21:30


English, 30.11.2020 21:30

![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0542/6727/5ea71.png)
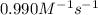
![[A]_o](/tpl/images/0542/6727/9caf5.png) = Initial concentration = 0.650 M
= Initial concentration = 0.650 M