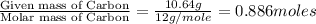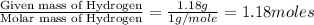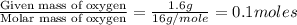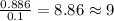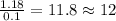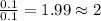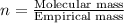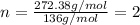Chemistry, 11.03.2020 03:31 choudharykaran7997
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon, hydrogen, and oxygen) produced 39.01 g CO2 and
10.65 g H2O. The molar mass of the unknown compound is 272.38 g/mol.
Find the molecular formula of the unknown compound.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
Combustion analysis of a 13.42-g sample of the unknown organic compound (which contains only carbon,...
Questions



History, 15.11.2019 02:31

Biology, 15.11.2019 02:31


Mathematics, 15.11.2019 02:31

Mathematics, 15.11.2019 02:31


Mathematics, 15.11.2019 02:31

Mathematics, 15.11.2019 02:31

History, 15.11.2019 02:31




Biology, 15.11.2019 02:31


Mathematics, 15.11.2019 02:31

Mathematics, 15.11.2019 02:31

History, 15.11.2019 02:31

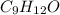 and
and 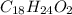
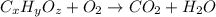
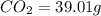
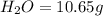
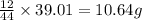 of carbon will be contained.
of carbon will be contained.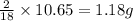 of hydrogen will be contained.
of hydrogen will be contained.