Chemistry, 11.03.2020 02:34 KillerSteamcar
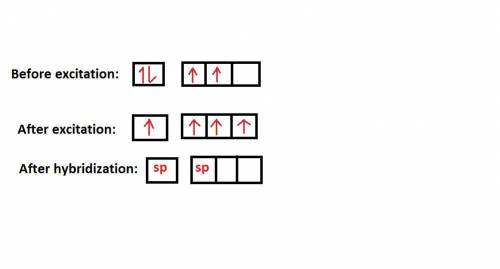
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon atom by sorting each orbital type. Consider the outer valence only.
Options include: sp orbitals, p orbitals, s orbitals
Put them in the following categories:
Zero One Two Three

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Consider a carbon atom that is sp hybridized. Indicate how many of each orbital exist on this carbon...
Questions

Mathematics, 13.10.2019 09:30

Health, 13.10.2019 09:30

Mathematics, 13.10.2019 09:30

Mathematics, 13.10.2019 09:30

Mathematics, 13.10.2019 09:30


Geography, 13.10.2019 09:30

Health, 13.10.2019 09:30

History, 13.10.2019 09:30



Mathematics, 13.10.2019 09:30


History, 13.10.2019 09:30



Biology, 13.10.2019 09:30



Biology, 13.10.2019 09:30

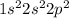 . This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.
. This means that in its neutral state it contains 2 electrons in its s-orbital and 2 electrons in its p-orbital.