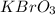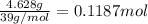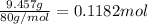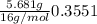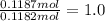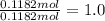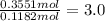Chemistry, 11.03.2020 03:07 vett072804
A sample of an ionic compound that is often used as a dough conditioner is analyzed and found to contain 4.628 g of potassium, 9.457 g of bromine, and 5.681 g of oxygen.
What is the empirical formula for this compound?
What is it chemical name?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
A sample of an ionic compound that is often used as a dough conditioner is analyzed and found to con...
Questions






Computers and Technology, 20.03.2021 03:10


Mathematics, 20.03.2021 03:10

Mathematics, 20.03.2021 03:10

Mathematics, 20.03.2021 03:10

Mathematics, 20.03.2021 03:10



Social Studies, 20.03.2021 03:10






Chemistry, 20.03.2021 03:10