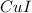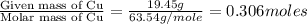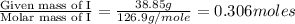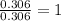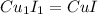Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3


Chemistry, 23.06.2019 05:30
What is the body’s main processing system? it uses input from various parts to control voluntary and involutiontary movement. it’s composed of two main parts-the brain and spinal cord. a. nbs b.cns c. ans d. pns
Answers: 1

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
A 19.45 gram sample of copper is heated in the presence of excess iodine. A metal iodide is formed w...
Questions

Social Studies, 10.07.2019 05:30


History, 10.07.2019 05:30




Mathematics, 10.07.2019 05:30












Geography, 10.07.2019 05:30