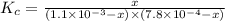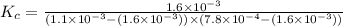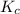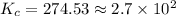Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is ma...

Chemistry, 11.03.2020 02:42 Chewbacka2020
Consider the following reaction:
Fe3+(aq)+SCN−(aq) <> FeSCN2+(aq)
A solution is made containing an initial [Fe3+] of 1.1 x 10^−3 M and an initial [SCN−] of 7.8 x 10^−4 M . At equilibrium, [FeSCN2+]= 1.6 x 10^−4 M .
Part A) Calculate the value of the equilibrium constant (Kc).
Express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
Questions

Health, 19.10.2021 06:30

Mathematics, 19.10.2021 06:30

Mathematics, 19.10.2021 06:30

History, 19.10.2021 06:30

History, 19.10.2021 06:30

Mathematics, 19.10.2021 06:40


Mathematics, 19.10.2021 06:40

Business, 19.10.2021 06:40

Spanish, 19.10.2021 06:40


Social Studies, 19.10.2021 06:40



Mathematics, 19.10.2021 06:40





History, 19.10.2021 06:40

 .
.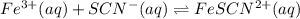
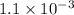
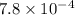 0
0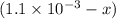
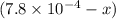 x
x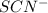 at equilibrium is given ,x=
at equilibrium is given ,x=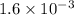
![K_c=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0542/1843/417f5.png)