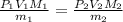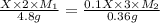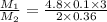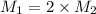Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume 3 L. The 2-L flask contains 4.8 g of gas, and the gas pressure is X atm. The 3-L flask contains 0.36 g of gas, and the gas pressure is 0.1X. Do the two gases have the same molar mass? If not, which contains the gas of higher molar mass?

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
Suppose you are given two flasks at the same temperature, one of volume 2 L and the other of volume...
Questions


Mathematics, 28.10.2021 02:50



Medicine, 28.10.2021 02:50

History, 28.10.2021 02:50


Computers and Technology, 28.10.2021 02:50


Spanish, 28.10.2021 02:50

Mathematics, 28.10.2021 02:50


Biology, 28.10.2021 02:50

Mathematics, 28.10.2021 02:50




History, 28.10.2021 02:50


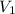 = 2 L,
= 2 L, 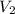 = 3 L,
= 3 L, 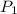 = X,
= X, 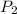 = 0.1 X,
= 0.1 X,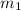 = 4.8 g,
= 4.8 g, 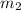 = 0.36 g,
= 0.36 g,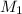 = ?,
= ?, 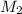 = ?
= ?