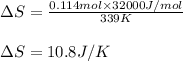Chemistry, 10.03.2020 23:02 Reebear8372
The heat of vaporization ΔΗν of tetrahydrofuran (C4H80) is 32.0 kJ/mol. Calculate the change in entropy AS when 8.2 g of tetrahydrofuran boils at 66.0 °C Be sure your answer contains a unit symbol and the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1


Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
The heat of vaporization ΔΗν of tetrahydrofuran (C4H80) is 32.0 kJ/mol. Calculate the change in entr...
Questions


Mathematics, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

English, 19.08.2019 01:30

Physics, 19.08.2019 01:30

English, 19.08.2019 01:30

English, 19.08.2019 01:30



Biology, 19.08.2019 01:30



Biology, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

Social Studies, 19.08.2019 01:30

History, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

English, 19.08.2019 01:30

Mathematics, 19.08.2019 01:30

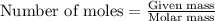
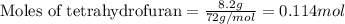
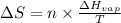
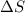 = Entropy change = ?
= Entropy change = ?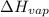 = enthalpy of vaporization = 32.0 kJ/mol = 32000 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of vaporization = 32.0 kJ/mol = 32000 J/mol (Conversion factor: 1 kJ = 1000 J)![66.0^oC=[66+273]K=339K](/tpl/images/0541/6883/b2b6e.png)