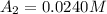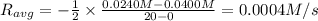Chemistry, 10.03.2020 22:12 chloesmolinski0909
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the concentration of the
reactant, the time interval, and the coefficient of the reactant.
Consider the following reaction:
2A+3B > 3C+2D
The concentrations of reactant A at three different time intervals are given. Use the following data to determine the average rate of reaction in terms of the disappearance of reactant A between time = 0 s and time = 20 s .
Time (s) 0 20 40
[A](M) 0.0400 0.0240 0.0180
Express your answer in molar concentration per second to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1


Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
(a) The rate of the reaction in terms of the "disappearance of reactant" includes the change in the...
Questions

Geography, 26.10.2020 22:40




Social Studies, 26.10.2020 22:40




Mathematics, 26.10.2020 22:40

History, 26.10.2020 22:40

Geography, 26.10.2020 22:40

Mathematics, 26.10.2020 22:40

History, 26.10.2020 22:40

Social Studies, 26.10.2020 22:40

Mathematics, 26.10.2020 22:40



Mathematics, 26.10.2020 22:40

Business, 26.10.2020 22:40

![R_{avg}=-\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/9f5c2.png)
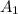 = initial concentration of reactant at
= initial concentration of reactant at 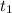 .
.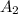 = Final concentration of reactant at
= Final concentration of reactant at 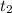 .
.![R_{avg}=-\frac{1}{2}\frac{[A]_2-[A]_1}{t_2-t_1}](/tpl/images/0541/5709/30159.png)
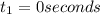 ) =
) = 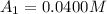
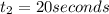 ) =
) =