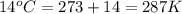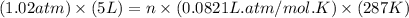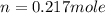Chemistry, 10.03.2020 20:35 mwilliams457
Calculate the amount of oxygen gas collected by the displacement of water at 14◦C if the atmospheric pressure is 790 Torr and the volume is 5 L. The vapor pressure of water at 14◦C is 12 Torr. 1. 0.217 mol 2. 0.00335 mol 3. 0.0184 mol 4. 4.46 mol 5. 0.224 mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 23.06.2019 01:00
Which substance—wood or silver—is the better thermal conductor? a thermal conductor is a material that requires very little heat energy to change its temperature. explain your answer.
Answers: 3
You know the right answer?
Calculate the amount of oxygen gas collected by the displacement of water at 14◦C if the atmospheric...
Questions

Chemistry, 01.07.2019 06:50

Social Studies, 01.07.2019 06:50






Social Studies, 01.07.2019 06:50

Spanish, 01.07.2019 06:50




History, 01.07.2019 06:50

History, 01.07.2019 06:50

Arts, 01.07.2019 06:50

Biology, 01.07.2019 07:00



Biology, 01.07.2019 07:00

Social Studies, 01.07.2019 07:00

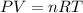
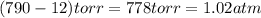 (1 atm = 760 torr)
(1 atm = 760 torr)