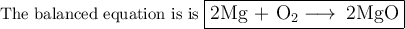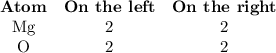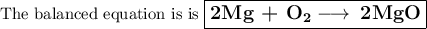Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.44 g of magnesium ribbon burns with 6.82 gof oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1


You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3...
Questions



English, 14.04.2021 22:00

History, 14.04.2021 22:00




Chemistry, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00


English, 14.04.2021 22:00


Biology, 14.04.2021 22:00

Mathematics, 14.04.2021 22:00



Mathematics, 14.04.2021 22:00



Mathematics, 14.04.2021 22:00