

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

You know the right answer?
What is the mole fraction of neon in a mixture that contains 0.628 g of helium, 11.491 g of neon, an...
Questions


Mathematics, 06.05.2020 05:15




Mathematics, 06.05.2020 05:15

History, 06.05.2020 05:15


Mathematics, 06.05.2020 05:15





Mathematics, 06.05.2020 05:15


Biology, 06.05.2020 05:15



Mathematics, 06.05.2020 05:15

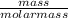
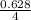 = 0.16moles
= 0.16moles 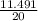 = 0.58moles
= 0.58moles 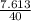 = 0.19moles
= 0.19moles 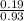 = 0.2
= 0.2

