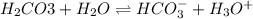Chemistry, 10.03.2020 20:00 sierranicasio
Which one would be expected to happen? If one of the buffers that contribute to pH stability in human blood is carbonic acid (H2CO3). Carbonic acid is a weak acid that when placed in an aqueous solution dissociates into a bicarbonate ion (HCO3-) and a hydrogen ion (H+). Thus, H2CO3 HCO3- + H+ If the pH of the blood increases.
A. A decrease in the concentration of HC03- and an increase in the concentration of both H2CO3 and H2O
B. An increase in the concentration of H2CO3 and a decrease in the concentration of H2O
C. An increase in the concentration of HCO3- and a decrease in the concentration of H2O
D. A decrease in the concentration of H2CO3 and an increase in the concentration of H2O
E. A decrease in the concentration of HCO3- and an increase in the concentration of H2O

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Which one would be expected to happen? If one of the buffers that contribute to pH stability in huma...
Questions


Mathematics, 19.10.2019 19:10

Mathematics, 19.10.2019 19:10


Chemistry, 19.10.2019 19:10


Mathematics, 19.10.2019 19:10

Chemistry, 19.10.2019 19:10




Chemistry, 19.10.2019 19:10






English, 19.10.2019 19:10


Mathematics, 19.10.2019 19:10