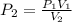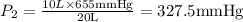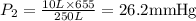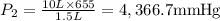Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2


Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
A 10.0-L balloon contains helium gas at a pressure of 655 mmHg. What is the new pressure, in mmHg, o...
Questions

Chemistry, 01.03.2021 07:00

Mathematics, 01.03.2021 07:00

English, 01.03.2021 07:00


Social Studies, 01.03.2021 07:00



English, 01.03.2021 07:00


Biology, 01.03.2021 07:00


Computers and Technology, 01.03.2021 07:00

Mathematics, 01.03.2021 07:00


English, 01.03.2021 07:00


Arts, 01.03.2021 07:00



Business, 01.03.2021 07:00

 10 = P2
10 = P2