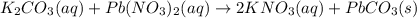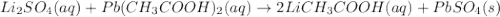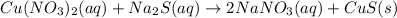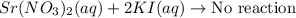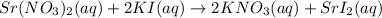Write a molecular equation for the precipitation reaction that occurs (if any) when the following solutions are mixed. If no reaction occurs, write NOREACTION.
Part A
potassium carbonate and lead(II) nitrate
Express your answer as a chemical equation. Enter NOREACTION if no reaction occurs. Identify all of the phases in your answer.
Part B
lithium sulfate and lead(II) acetate
Express your answer as a chemical equation. Enter NOREACTION if no reaction occurs. Identify all of the phases in your answer.
Part C
copper(II) nitrate and sodium sulfide
Express your answer as a chemical equation. Enter NOREACTION if no reaction occurs. Identify all of the phases in your answer.
Part D
strontium nitrate and potassium iodide
Express your answer as a chemical equation. Enter NOREACTION if no reaction occurs. Identify all of the phases in your answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 23.06.2019 01:00
Who examines and coordinates the cleanup of polluted sites?
Answers: 2

Chemistry, 23.06.2019 01:00
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1

Chemistry, 23.06.2019 02:00
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1
You know the right answer?
Write a molecular equation for the precipitation reaction that occurs (if any) when the following so...
Questions

Mathematics, 19.10.2019 12:50


English, 19.10.2019 12:50


Mathematics, 19.10.2019 12:50


English, 19.10.2019 12:50


English, 19.10.2019 12:50


Mathematics, 19.10.2019 12:50








Spanish, 19.10.2019 12:50

Mathematics, 19.10.2019 12:50