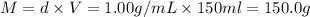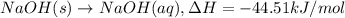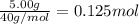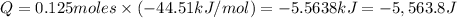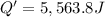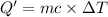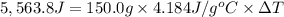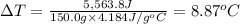Chemistry, 10.03.2020 19:01 gooberthebear8955
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in temperature of a coffee cup calorimeter containing 150 ml of water when 5.00 g NaOH (40.00 g/mol) is added to the container. You may assume that the solution has the same specific heat and density as water.
NaOH(s) → NaOH(aq) ΔH =-44.51 KJ
a. +8.87°C
b. +2.70 C
c. 2.70°C
d. 8.87°C

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1


Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
The enthalpy of solution (dissolving) of sodium hydroxide is given below. Determine the change in te...
Questions

Mathematics, 26.05.2020 22:00



Mathematics, 26.05.2020 22:00



Mathematics, 26.05.2020 22:00



History, 26.05.2020 22:00




Mathematics, 26.05.2020 22:00