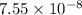Chemistry, 10.03.2020 19:01 topangabraith
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (Kc) at a certain temperature, are given below. reaction (1): N2(g) + O2(g) equilibrium reaction arrow 2 NO(g); Kc = 2.59e-31 reaction (2): N2(g) + 1/2 O2(g) equilibrium reaction arrow N2O(g); Kc = 3.31e-24 Using this set of data, determine the equilibrium constant for the following reaction, at the same temperature. reaction (3): N2O(g) + 1/2 O2(g) equilibrium reaction arrow 2 NO(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

You know the right answer?
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (K...
Questions

Chemistry, 17.10.2019 09:01


Mathematics, 17.10.2019 09:01




Health, 17.10.2019 09:01

Biology, 17.10.2019 09:01



Mathematics, 17.10.2019 09:01

Medicine, 17.10.2019 09:01


Mathematics, 17.10.2019 09:01


English, 17.10.2019 09:01




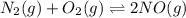
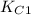 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}][O_{2}]}](/tpl/images/0541/2546/adeca.png)
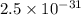
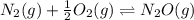
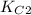 of this reaction is as follows.
of this reaction is as follows.![\frac{[N_{2}O]}{[N_{2}][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/7b620.png)
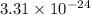
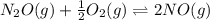
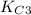 of this reaction is as follows.
of this reaction is as follows.![\frac{[NO]^{2}}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/c0a44.png)
![\frac{[NO]^{2}}{[N_{2}][O_{2}]} \times \frac{[N_{2}][O_{2}]}{[N_{2}O][O_{2}]^{\frac{1}{2}}}](/tpl/images/0541/2546/93fb5.png)
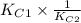
![[tex]2.5 \times 10^{-31} \times \frac{1}{3.31 \times 10^{-24}}](/tpl/images/0541/2546/55cbe.png)