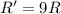Chemistry, 10.03.2020 19:06 ryliekarenbowers
By what factor does the rate change in each of the following cases (assuming constant temperature)?
Factor (enter as decimal if <1)
(a) A reaction is first order in reactant A, and [A] is doubled.
(b) A reaction is second order in reactant B, and [B] is halved.
(c) A reaction is second order in reactant C, and [C] is tripled.

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1

Chemistry, 23.06.2019 19:40
Alab technician needs to create 570.0 milliliters of a 2.00 m solution of magnesium chloride (mgcl2). to make this solution, how many grams of magnesium chloride does the technician need? refer to the periodic table for . express your answer to three significant figures.
Answers: 3
You know the right answer?
By what factor does the rate change in each of the following cases (assuming constant temperature)?...
Questions









Mathematics, 02.08.2019 22:20

Health, 02.08.2019 22:20

Spanish, 02.08.2019 22:20

Mathematics, 02.08.2019 22:20





Computers and Technology, 02.08.2019 22:20



Mathematics, 02.08.2019 22:20

![R=k[A]^1](/tpl/images/0541/2780/9975f.png)
![R'=k[2A]^1](/tpl/images/0541/2780/53afe.png)
![R'=2k[A]](/tpl/images/0541/2780/37f63.png)
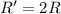
![R=k[B]^2](/tpl/images/0541/2780/92409.png)
![R'=k[\frac{B}{2}]^2](/tpl/images/0541/2780/d1975.png)
![R'=\frac{1}{4}k[B]^2](/tpl/images/0541/2780/1571d.png)
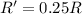
![R=k[C]^2](/tpl/images/0541/2780/01b2f.png)
![R'=k[3C]^2](/tpl/images/0541/2780/92d86.png)
![R'=9k[C]^2](/tpl/images/0541/2780/6f960.png)