

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
Reaction rate is expressed in terms of changes in the concentration of reactants and products. Write...
Questions

Mathematics, 11.05.2021 19:30

Social Studies, 11.05.2021 19:30

Mathematics, 11.05.2021 19:30

Mathematics, 11.05.2021 19:30




Mathematics, 11.05.2021 19:30





Mathematics, 11.05.2021 19:30







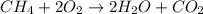
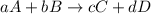
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0541/3019/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0541/3019/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0541/3019/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0541/3019/d4b94.png)
![Rate=-\frac{d[CH_4]}{dt}=-\frac{1}{2}\frac{d[O_2]}{dt}=+\frac{1}{2}\frac{d[H_2O]}{dt}=+\frac{d[CO_2]}{dt}](/tpl/images/0541/3019/dfd60.png)


