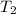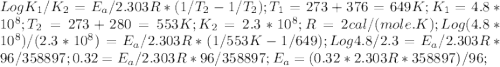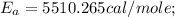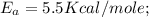The rate constant k for a certain reaction is measured at two different temperatures:
te...

Chemistry, 10.03.2020 18:49 kkwolfcityouwc96
The rate constant k for a certain reaction is measured at two different temperatures:
temperature k
376.0 °c 4.8 x 108
280.0 °C 2.3 x 10 8
Assuming the rate constant obeys the Arrhenius equation, calculate the activation energy Ea for this reaction.
Round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Questions


Mathematics, 18.05.2020 10:57


Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57




Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57

Mathematics, 18.05.2020 10:57



Mathematics, 18.05.2020 11:57

Chemistry, 18.05.2020 11:57

History, 18.05.2020 11:57

Mathematics, 18.05.2020 11:57

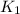 and
and 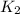 at temperature
at temperature 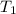 and
and