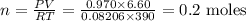Chemistry, 10.03.2020 18:38 sahaitong1844
An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed and 6.60 L of oxygen gas was released at a pressure of 0.970 atm and
390.0 K. What was the initial weight of mercury oxide in the sample? (1 point)
1) 48.91 grams
2) 64.32 grams
3) 78.32 grams
4) 86.6 grams

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1


Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
An unknown amount of mercury (II) oxide was decomposed in the lab. Mercury metal
was formed an...
was formed an...
Questions





Computers and Technology, 07.01.2020 02:31

Social Studies, 07.01.2020 02:31

History, 07.01.2020 02:31



Mathematics, 07.01.2020 02:31






Mathematics, 07.01.2020 02:31



Mathematics, 07.01.2020 02:31