
Chemistry, 10.03.2020 17:19 whitethunder05
What mass of butane in grams is necessary to produce 1.5×103 kJ1.5×103 kJ of heat? What mass of CO2CO2 is produced? Assume the reaction to be as follows: C4H10(g)+132O2(g)→4CO2(g)+5H2O(g),Δ Hrxn=−2658 kJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

You know the right answer?
What mass of butane in grams is necessary to produce 1.5×103 kJ1.5×103 kJ of heat? What mass of CO2C...
Questions










Physics, 31.03.2020 00:00

English, 31.03.2020 00:01


Mathematics, 31.03.2020 00:01







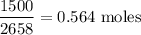 of butane reacted
of butane reacted 

