20 mL of an approximately 10% aqueous solution of
ethylamine, CH3CH2NH2, is titrated with 0.30...

Chemistry, 10.03.2020 09:11 valenciafaithtorres
20 mL of an approximately 10% aqueous solution of
ethylamine, CH3CH2NH2, is titrated with 0.3000 M
aqueous HCl. Which indicator would be most suitable
for this titration? The pKa of CH3CH2NH3
+ is 10.75.
(A) Thymol blue, color change from pH = 1.2 to 2.8
(B) Bromocresol green, color change from pH = 4.0 to
5.6
(C) Phenolphthalein, color change from pH = 8.0 to 10.0
(D) Alizarin yellow R, color change from pH = 10.0 to
12.0

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 09:10
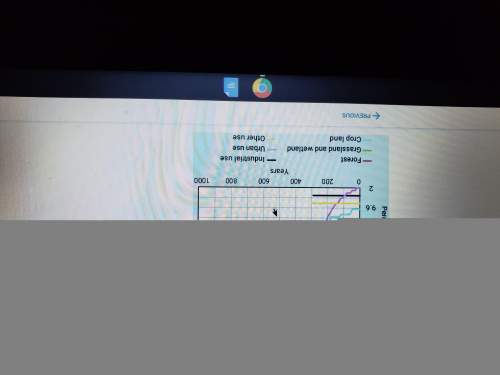
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

You know the right answer?
Questions



Mathematics, 16.04.2021 04:00



History, 16.04.2021 04:00


Mathematics, 16.04.2021 04:00


Mathematics, 16.04.2021 04:00

Social Studies, 16.04.2021 04:00



Arts, 16.04.2021 04:00

English, 16.04.2021 04:00

History, 16.04.2021 04:00


Arts, 16.04.2021 04:00

Geography, 16.04.2021 04:00

Biology, 16.04.2021 04:00