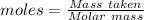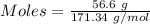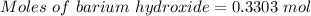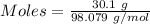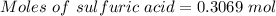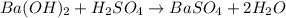For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of sulfuric acid. barium hydroxide (aq) + sulfuric acid (aq) barium sulfate (s) + water (l) What is the maximum amount of barium sulfate that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? grams

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
11) burning your hand when accidentally touching a hot plate is an example of which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 2

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
For the following reaction, 56.6 grams of barium hydroxide are allowed to react with 30.1 grams of s...
Questions

Mathematics, 03.02.2021 01:10



Mathematics, 03.02.2021 01:10

Mathematics, 03.02.2021 01:10

Mathematics, 03.02.2021 01:10



Mathematics, 03.02.2021 01:10

Biology, 03.02.2021 01:10


Spanish, 03.02.2021 01:10




English, 03.02.2021 01:10

History, 03.02.2021 01:10

Mathematics, 03.02.2021 01:10

Mathematics, 03.02.2021 01:10

 .
.