
Chemistry, 10.03.2020 08:05 andreanaapollon6337
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon by mass. What is the empirical formula of the compound

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1


Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1
You know the right answer?
A gaseous compound containing carbon and hydrogen was analyzed and found to consist of 83.65% carbon...
Questions

Mathematics, 10.12.2020 08:50


English, 10.12.2020 08:50

Engineering, 10.12.2020 08:50


Mathematics, 10.12.2020 08:50



History, 10.12.2020 08:50



Biology, 10.12.2020 08:50


History, 10.12.2020 08:50

Geography, 10.12.2020 08:50



Biology, 10.12.2020 08:50

Chemistry, 10.12.2020 08:50

Spanish, 10.12.2020 08:50

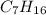
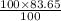 is 83.65 gram
.
is 83.65 gram
.

