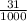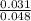Chemistry, 10.03.2020 08:16 jordandabrat
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion concentration [H+]. Show all work.
B. Calculate the pH. Show all work.
C. Is this solution an acid, base, or neutral? You must explain how you know.
2) Calculate the molarity of a solution prepared by dissolving 195 g of sodium chloride (NaCl) in enough water to make 2.8 liters of solution.
3) A student takes 152 mL of a 3.0M HCl solution and places it into a beaker. The student then adds enough water to the beaker to make 750 mL. Calculate the molarity of this new solution. Show all work.
4) A student started a titration by placing 20.0 mL of 0.24 M NaOH into a flask with 3 drops of phenolphthalein. The student then titrated to the endpoint using 31.0 mL of HCl. Calculate the concentration (molarity) of the HCl that the student used. Show all work for credit.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
1) You have an aqueous solution where [OH-] = 1 x 10-4 mol/L.
A. Calculate the hydrogen ion co...
A. Calculate the hydrogen ion co...
Questions


Mathematics, 17.06.2021 01:00







Biology, 17.06.2021 01:00




Mathematics, 17.06.2021 01:00

History, 17.06.2021 01:00

Mathematics, 17.06.2021 01:00





Mathematics, 17.06.2021 01:00

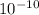 x is the hydrogen ion concentration.
x is the hydrogen ion concentration.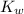 = [
= [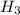 O+] [OH]-
O+] [OH]- 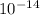
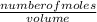
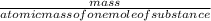
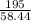 ( atomic weight of NaCl is 58.44 gm/mole)
( atomic weight of NaCl is 58.44 gm/mole)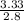
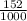 = M2 ×
= M2 × 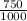
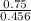
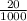 = Macid x
= Macid x