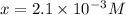Chemistry, 10.03.2020 08:25 ballin2126
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium concentration of H3O+ in the solution if the initial concentration of C6H5COOH is 7.0×10−2 M .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium co...
Questions

English, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

History, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50


Mathematics, 27.05.2021 20:50

Chemistry, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50


Mathematics, 27.05.2021 20:50

Mathematics, 27.05.2021 20:50

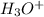 in the solution is,
in the solution is, 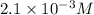
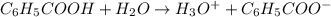
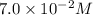
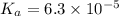
![K_a=\frac{[H_3O^+][C_6H_5COO^-]}{[C_6H_5COOH]}](/tpl/images/0540/6257/de276.png)
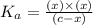
![6.3\times 10^{-5}=\frac{(x)\times (x)}{[(7.0\times 10^{-2})-x]}](/tpl/images/0540/6257/1bf1d.png)