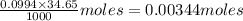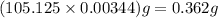Chemistry, 10.03.2020 06:47 payshencec21
A student titrated a weak acid with 0.0994 M NaOH solution. It took the student 34.65mL of NaOH to reach the equivalence point. How many grams of weak acid did the student use for titration, if molar mass of the weak acid was 105.125 g/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
A student titrated a weak acid with 0.0994 M NaOH solution. It took the student 34.65mL of NaOH to r...
Questions

English, 05.07.2019 02:30

Chemistry, 05.07.2019 02:30






Social Studies, 05.07.2019 02:30



Health, 05.07.2019 02:30

English, 05.07.2019 02:30

Mathematics, 05.07.2019 02:30

Social Studies, 05.07.2019 02:30

Mathematics, 05.07.2019 02:30

Mathematics, 05.07.2019 02:30

Spanish, 05.07.2019 02:30

English, 05.07.2019 02:30