
Chemistry, 10.03.2020 06:31 Halieyrobinson3003
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank is at 2000 psia and 80°F. The oxygen is removed from the tank slowly enough that the temperature in the tank remains at 80°F. After two weeks, the pressure in the tank is 100 psia. Determine the mass of oxygen used in lbm. Also determine the total heat transfer to the tank in Btu. Treat the oxygen as an ideal gas with constant specific heats at 80°F.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 22:00
What mass of glucose is produced when 54g of water react with carbon dioxide
Answers: 1
You know the right answer?
Oxygen is supplied to a medical facility from a 30 ft3 compressed oxygen tank. Initially, the tank i...
Questions





Mathematics, 05.12.2019 21:31








Social Studies, 05.12.2019 21:31

Mathematics, 05.12.2019 21:31


Mathematics, 05.12.2019 21:31

History, 05.12.2019 21:31



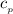 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R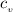 = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R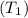 in the tank is given as 80°F.
in the tank is given as 80°F.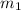 in the tank from the ideal gas equation can be calculated as:
in the tank from the ideal gas equation can be calculated as: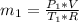
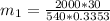
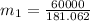
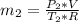
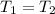 = 540 R
= 540 R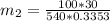
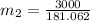
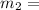 16.57 lbm
16.57 lbm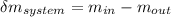
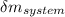 = change in the initial mass and final mass of the oxygen in the tank
= change in the initial mass and final mass of the oxygen in the tank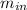 = the inlet mass of the oxygen
= the inlet mass of the oxygen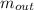 = the outlet mass of the oxygen
= the outlet mass of the oxygen
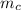 (i.e amount of oxygen used in the system)
(i.e amount of oxygen used in the system)
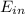 -
- 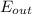 =
= 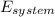
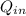 -
-  =
= 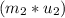 -
- 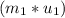
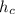 = specific enthalpy of the oxygen used
= specific enthalpy of the oxygen used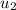 = specific internal energy of the final mass
= specific internal energy of the final mass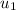 = specific internal energy of the initial mass
= specific internal energy of the initial mass
 &
& 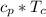
![[m_2*(c_v*T_2)]-[m_1*(c_v*T_1)]+[m_c*(c_p*T_c)]](/tpl/images/0540/2343/fb1ed.png)
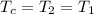
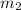 = 16.57 lbm
= 16.57 lbm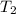 = 540 R
= 540 R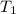 = 540 R
= 540 R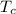 = 540 R
= 540 R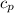 = 0.219 Btu/lbm.R
= 0.219 Btu/lbm.R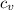 = 0.157 Btu/lbm.R
= 0.157 Btu/lbm.R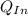 = [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]
= [16.57×(0.157×540)]-[331.38×(0.157×540)]+[314.81×(0.219×540)]

