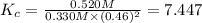Chemistry, 10.03.2020 05:58 emilie3849
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.800 M , and [C] = 0.350 M . The following reaction occurs and equilibrium is established: A+2B⇌C At equilibrium, [A] = 0.330 M and [C] = 0.520 M . Calculate the value of the equilibrium constant, Kc.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1


Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1

You know the right answer?
A mixture initially contains A, B, and C in the following concentrations: [A] = 0.500 M , [B] = 0.80...
Questions


Advanced Placement (AP), 11.10.2019 20:30



Physics, 11.10.2019 20:30


Mathematics, 11.10.2019 20:30


English, 11.10.2019 20:30


Chemistry, 11.10.2019 20:30



Mathematics, 11.10.2019 20:30

Mathematics, 11.10.2019 20:30

History, 11.10.2019 20:30

Arts, 11.10.2019 20:30

Mathematics, 11.10.2019 20:30


English, 11.10.2019 20:30

![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0540/1386/240ef.png)