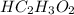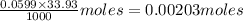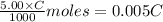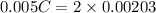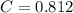Chemistry, 10.03.2020 05:58 ayoismeisalex
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M Ca(OH)2, and 33.93 mL of the Ca(OH)2 solution is required to reach the equivalence point. What is the molarity of the acetic acid? Group of answer choices

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
How many ions that have a +1 charge will bond with an ion that has a -2 charge
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
A 5.00 mL sample of vinegar, an aqueous solution of acetic acid (HC2H3O2), is titrated with 0.0599 M...
Questions








English, 09.10.2019 09:30

Arts, 09.10.2019 09:30


English, 09.10.2019 09:30


Health, 09.10.2019 09:30

Mathematics, 09.10.2019 09:30


Mathematics, 09.10.2019 09:30

Mathematics, 09.10.2019 09:30


History, 09.10.2019 09:30

Chemistry, 09.10.2019 09:30

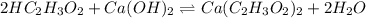
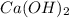 neutralizes 2 moles of
neutralizes 2 moles of