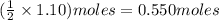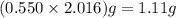Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Consider the balanced equation. 2HCl + Mg MgCl2 + H2 If 40.0 g of HCl react with an excess of magnes...
Questions

Mathematics, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10

English, 21.05.2021 05:10

Biology, 21.05.2021 05:10


Law, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10



Mathematics, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10

Mathematics, 21.05.2021 05:10


Social Studies, 21.05.2021 05:10



Mathematics, 21.05.2021 05:20

Mathematics, 21.05.2021 05:20

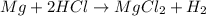
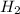 .
.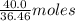 of HCl = 1.10 moles of HCl
of HCl = 1.10 moles of HCl