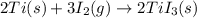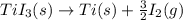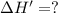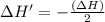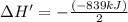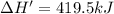Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 04:20
Which activity describes an application of topographic maps? check all that apply. recreation, such as camping and hiking engineering, such as the construction of roads and buildings science, such as mapping stars in the sky business, such as analyzing population centers science, such as analyzing surface features
Answers: 1
You know the right answer?
The enthalpy change for the reaction of titanium metal with gaseous iodine is given by the following...
Questions

Health, 25.07.2019 07:30



Mathematics, 25.07.2019 07:30

Health, 25.07.2019 07:30

Computers and Technology, 25.07.2019 07:30


Health, 25.07.2019 07:30


Business, 25.07.2019 07:30

Mathematics, 25.07.2019 07:30



Biology, 25.07.2019 07:30

Arts, 25.07.2019 07:30





Mathematics, 25.07.2019 07:30