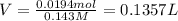Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations would be expected to neutralize. Assume complete neutralization.
a. A tablet containting 350 mg Al(OH)3 and 250 mg Mg(OH)2.
b. A tablet containing 970 mg of CaCO3.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Calculate the maximum volume (in mL) of 0.143 M HCl that each of the following antacid formulations...
Questions

Mathematics, 06.12.2019 14:31

History, 06.12.2019 14:31

History, 06.12.2019 14:31




English, 06.12.2019 14:31



Health, 06.12.2019 14:31


Biology, 06.12.2019 14:31



Mathematics, 06.12.2019 14:31


Biology, 06.12.2019 14:31


Advanced Placement (AP), 06.12.2019 14:31

Mathematics, 06.12.2019 14:31

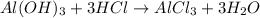
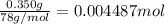
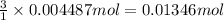 of HCl.
of HCl.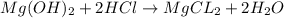
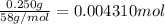
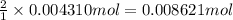 of HCl.
of HCl.
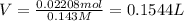
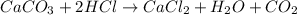
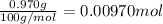
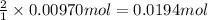 of HCl.
of HCl.