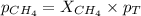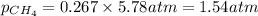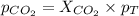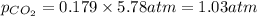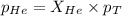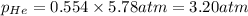Chemistry, 10.03.2020 04:42 erikap0889
Determine the mole fractions and partial pressures of CO2, CH4, and He in a sample of gas that contains 1.20 moles of CO2, 1.79 moles of CH4, and 3.71 moles of He, and in which the total pressure is 5.78 atm.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Determine the mole fractions and partial pressures of CO2, CH4, and He in a sample of gas that conta...
Questions

Mathematics, 05.03.2022 03:10






Geography, 05.03.2022 03:20


Computers and Technology, 05.03.2022 03:20





Physics, 05.03.2022 03:20

Business, 05.03.2022 03:20


Mathematics, 05.03.2022 03:20



Mathematics, 05.03.2022 03:20

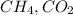 and
and 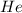 gases are, 0.267, 0.179, 0.554 and 1.54, 1.03 and 3.20 atm respectively.
gases are, 0.267, 0.179, 0.554 and 1.54, 1.03 and 3.20 atm respectively.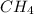 = 1.79 mole
= 1.79 mole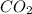 = 1.20 mole
= 1.20 mole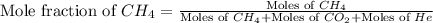
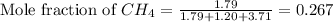
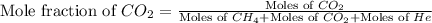
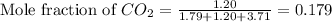
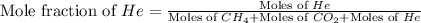
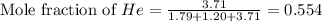
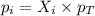
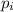 = partial pressure of gas
= partial pressure of gas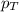 = total pressure of gas = 5.78 atm
= total pressure of gas = 5.78 atm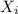 = mole fraction of gas
= mole fraction of gas