

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3


Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
If 0.953 g of copper was actually recovered at the end of the entire experiment, what would be the p...
Questions



Mathematics, 07.02.2021 06:50



Mathematics, 07.02.2021 06:50


Social Studies, 07.02.2021 06:50

History, 07.02.2021 06:50




Mathematics, 07.02.2021 07:00


Health, 07.02.2021 07:00




Mathematics, 07.02.2021 07:00

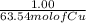
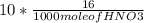
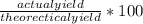
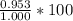 %
%


