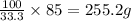Chemistry, 10.03.2020 04:47 morenodonaldo762
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3% w/w solution of acetic acid in ethanol. Calculate the mass of solution the student should use. If there's not enough solution, press the "No solution" button. Be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
A chemistry student needs 85.0g of acetic acid for an experiment. She has available 0.20kg of a 33.3...
Questions



SAT, 31.05.2021 04:00


Mathematics, 31.05.2021 04:00


Geography, 31.05.2021 04:00





Computers and Technology, 31.05.2021 04:00






Mathematics, 31.05.2021 04:00


Chemistry, 31.05.2021 04:00