
Chemistry, 10.03.2020 04:49 ngmasuku3115
Be sure to answer all parts. The equilibrium constant, Kc, for the formation of nitrosyl chloride from nitric oxide and chlorine,2NO(g) + Cl2(g) ⇌ 2NOCl(g)is 6.5 ×104 at 35°C. Calculate KP for this reaction, and determine whether the reaction will proceed to the right or to the left to achieve equilibrium when the starting pressures are PNO = 1.01 atm, PCl2 = 0.42 atm, and PNOCl = 1.76 atm.×10(Enter your answer in scientific notation.)reaction will proceed to the rightreaction is at equilibriumreaction will proceed to the left

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 06:30
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Be sure to answer all parts. The equilibrium constant, Kc, for the formation of nitrosyl chloride fr...
Questions

Mathematics, 14.11.2020 01:50


Mathematics, 14.11.2020 01:50



English, 14.11.2020 01:50



Chemistry, 14.11.2020 01:50


Mathematics, 14.11.2020 01:50

History, 14.11.2020 01:50


Mathematics, 14.11.2020 01:50

Mathematics, 14.11.2020 01:50

Mathematics, 14.11.2020 01:50



English, 14.11.2020 01:50

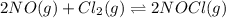
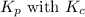 is given by the formula:
is given by the formula: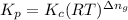
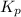 = equilibrium constant in terms of partial pressure = ?
= equilibrium constant in terms of partial pressure = ?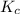 = equilibrium constant in terms of concentration =
= equilibrium constant in terms of concentration = 
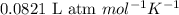
![35^oC=[35+273]K=308K](/tpl/images/0540/0407/bab2f.png)
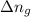 = change in number of moles of gas particles =
= change in number of moles of gas particles = 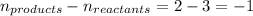
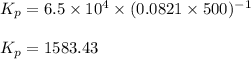
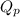 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.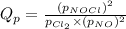
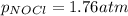
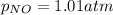
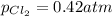
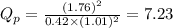
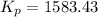
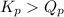 ; the reaction is product favored.When
; the reaction is product favored.When 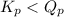 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 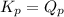 ; the reaction is in equilibrium
; the reaction is in equilibrium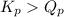 , the reaction will be favoring product side.
, the reaction will be favoring product side.

