
Chemistry, 10.03.2020 04:52 Katie123amazing
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are constant over the temperature interval at their values at 298.15 K. Molar heat capacities of NO(g), N2(g), and O2(g) at 298.15 K are 29.86, 29.13, and 29.38 J⋅K−1⋅mol−1. The standard enthalpy of formation of NO(g) is 91.3 kJ⋅mol−1 at 298.15 K.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1



Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1
You know the right answer?
Calculate ΔH∘f for NO(g) at 435 K, assuming that the heat capacities of reactants and products are c...
Questions


Mathematics, 30.08.2019 23:00


Social Studies, 30.08.2019 23:00


Mathematics, 30.08.2019 23:00

Mathematics, 30.08.2019 23:00

Geography, 30.08.2019 23:00

Biology, 30.08.2019 23:00




World Languages, 30.08.2019 23:00

Geography, 30.08.2019 23:00

History, 30.08.2019 23:00





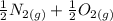 ------>
------>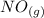
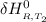 =
= 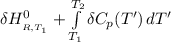
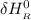 = enthalpy of reaction
= enthalpy of reaction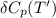 = the difference in the heat capacities of the products and the reactants.
= the difference in the heat capacities of the products and the reactants.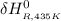 =
= 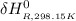

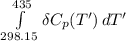
![1(91300 J.mol^{-1} ) +\int\limits^{435}_{298.15} [{(29.86)-\frac{1}{2}(29.38)-\frac{1}{2}29.13}]J.K^{-1}.mol^{-1} \, dT'](/tpl/images/0540/0534/3b971.png)


