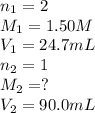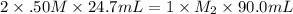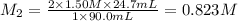Chemistry, 10.03.2020 04:01 marygatewell385
A volume of 90.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard solution of sulfuric acid (H2SO4H2SO4). What was the molarity of the KOHKOH solution if 24.7 mLmL of 1.50 MM H2SO4H2SO4 was needed

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Scientist wants to react a barium chloride salt with an acid. to speed up the reaction, the scientist crushes the barium chloride salt until it is a fine powder. this speeds up the reaction by a. increasing the ph of the salt. b. increasing the surface area of the salt. c. increasing the pressure of the salt. d. increasing the temperature of the salt. im thinking more a not 100% sure
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
You know the right answer?
A volume of 90.0 mLmL of aqueous potassium hydroxide (KOHKOH) was titrated against a standard soluti...
Questions

History, 28.01.2021 18:50




History, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50



English, 28.01.2021 18:50



Computers and Technology, 28.01.2021 18:50




Mathematics, 28.01.2021 18:50


Mathematics, 28.01.2021 18:50

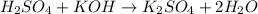 (Neutralization reaction)
(Neutralization reaction)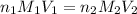
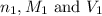 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 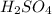
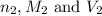 are the n-factor, molarity and volume of base which is KOH.
are the n-factor, molarity and volume of base which is KOH.