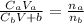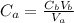Chemistry, 10.03.2020 04:00 dsaefong00
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to the stoi- chiometric point. Determine the molar concentration of the weak acid solution. Express your answer to the correct number of significant figures.

Answers: 3


Another question on Chemistry



Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 15:30
Amole, which is a unit in chemistry, contains 6.02 x 1023 atoms or particles. how many zeroes follow the 2 when this number is written out in standard form? a) 21 b) 23 c) 24 d) 25
Answers: 1
You know the right answer?
A 23.74-mL volume of 0.0981 M NaOH was used to titrate 25.0 mL of a weak monoprotic acid solution to...
Questions

English, 22.08.2019 22:30






Computers and Technology, 22.08.2019 22:30

Chemistry, 22.08.2019 22:30

Mathematics, 22.08.2019 22:30

Biology, 22.08.2019 22:30




English, 22.08.2019 22:30




Social Studies, 22.08.2019 22:30

Mathematics, 22.08.2019 22:30

Physics, 22.08.2019 22:30