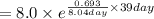Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
Iodine-131 is a beta emitter used as a tracer in radio immunoassays biological systems. It follows f...
Questions

Mathematics, 04.04.2020 07:28

History, 04.04.2020 07:28





Medicine, 04.04.2020 07:28


Mathematics, 04.04.2020 07:28











![[A_o]=8.0 g](/tpl/images/0539/8436/6a79c.png)

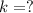

![[A]=[A_o]\times e^{-kt}](/tpl/images/0539/8436/abdec.png)
![[A]=8.0\times e^{-\frac{0.693}{t_{1/2}\times t}](/tpl/images/0539/8436/554b5.png)