
Chemistry, 10.03.2020 03:04 ijustneedhelp29
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D (aq) Kc = 178. If initially the concentrations of the chemical species are as follows: [A]o = 0.364 M [B]o = 0.170 M [C]o = 0.132 M [D]o = 0.932 M to which direction will the reaction run to reach equilibrium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

You know the right answer?
Consider the following equilibrium reaction at a given temperature: A (aq) + 3 B (aq) ⇌ C (aq) + 2 D...
Questions


Mathematics, 21.09.2021 14:00



Mathematics, 21.09.2021 14:00





Mathematics, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00



Mathematics, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00

Chemistry, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00


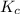 is the constant of a certain reaction at equilibrium while
is the constant of a certain reaction at equilibrium while 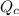 is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.
is the quotient of activities of products and reactants at any stage other than equilibrium of a reaction.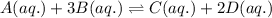
![Q_c=\frac{[D]^2[C]}{[A][B}^3}](/tpl/images/0539/7435/19a81.png)
![[A]_o=0.364M](/tpl/images/0539/7435/6e4ee.png)
![[B]_o=0.170M](/tpl/images/0539/7435/df462.png)
![[C]_o=0.132M](/tpl/images/0539/7435/924d4.png)
![[D]_o=0.932M](/tpl/images/0539/7435/7f66b.png)
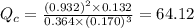

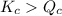 ; the reaction is product favored.When
; the reaction is product favored.When 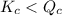 ; the reaction is reactant favored.When
; the reaction is reactant favored.When 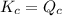 ; the reaction is in equilibrium.
; the reaction is in equilibrium.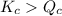 , the reaction will be favoring product side.
, the reaction will be favoring product side.

