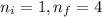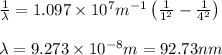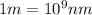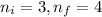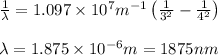Chemistry, 10.03.2020 02:57 urstruulyemily
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible region corresponding to the Balmer series in other series emmission lines are present in different regions of the eletromagnetic spectrum
Calculate the wavelenth of the n=4 to n=1 and the n=4 to n=3 transitions. Indicate in which regions of the electromagnetic spectrum these transitions would occur.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
In the emission spectrum of hydrogen the transitions observed in this experiment are in the visible...
Questions







Social Studies, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00


Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00

Mathematics, 26.02.2021 01:00



English, 26.02.2021 01:00

History, 26.02.2021 01:00



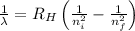
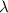 = Wavelength of radiation
= Wavelength of radiation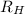 = Rydberg's Constant =
= Rydberg's Constant = 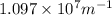
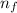 = Higher energy level
= Higher energy level 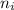 = Lower energy level
= Lower energy level