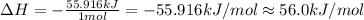Chemistry, 10.03.2020 01:44 tobyhollingsworth178
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g of water to yield the following reaction: NaOH + HBr → Na+(aq) + Br-(aq) + H2O(l) After mixing the temperature rises to 88.5 oC. Calculate the change in enthalpy of this reaction. Specific heat of the solution = 4.184 J/(g oC) State your answer in kJ with 3 significant figures. Don't forget to enter the unit behind the numerical answer. The molecular weight of NaOH is 40.0 g/mol, and the molecular weight of HBr is 80.9 g/mol. ΔH =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 1 mol NaOH and 1 mol HBr initially at 28 oC (Celsius) are mixed in 100g...
Questions

Physics, 13.02.2021 05:40





Mathematics, 13.02.2021 05:40

Mathematics, 13.02.2021 05:40


Mathematics, 13.02.2021 05:40

Biology, 13.02.2021 05:40

Spanish, 13.02.2021 05:40

Spanish, 13.02.2021 05:40

Mathematics, 13.02.2021 05:40

English, 13.02.2021 05:40

Arts, 13.02.2021 05:40

Mathematics, 13.02.2021 05:40


History, 13.02.2021 05:40

Mathematics, 13.02.2021 05:40

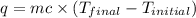
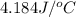
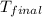 = final temperature =
= final temperature = 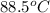
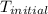 = initial temperature =
= initial temperature = 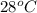
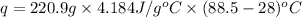
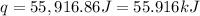 ( J = 0.001 kJ)
( J = 0.001 kJ)
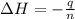
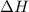 = enthalpy change = ?
= enthalpy change = ?