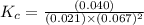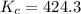Chemistry, 10.03.2020 01:48 jennyhoang4330
A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3OH), a compound considered an alternative fuel to gasoline. Under equilibrium conditions at 540.2 K, [H2] = 0.067 mol [CO]=0.021 mol/L and [CH3OH]=0.040 mol/L what is the value of Kc for this reaction at 505.0 K?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
You know the right answer?
A mixture of gaseous CO and H2, called synthesis gas, is used commercially to prepare methanol (CH3O...
Questions


Mathematics, 18.06.2021 16:00

Chemistry, 18.06.2021 16:00







Mathematics, 18.06.2021 16:00



Arts, 18.06.2021 16:00

History, 18.06.2021 16:00




Business, 18.06.2021 16:00


Mathematics, 18.06.2021 16:00

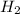 at equilibrium = 0.067 mol
at equilibrium = 0.067 mol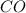 at equilibrium = 0.021 mol
at equilibrium = 0.021 mol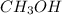 at equilibrium = 0.040 mol
at equilibrium = 0.040 mol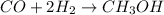
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0539/5815/4cf94.png)